Digital verification is transforming how we design, validate, and certify industrial processes and equipment. Traditionally, verification has relied on physical testing, documentation, and manual procedures, but digital tools now make it possible to demonstrate compliance virtually faster, safer, and more transparently.
This seminar focuses on digital verification of processes and equipment with emphasis on Good Manufacturing Practice (GMP) requirements, certification procedures, and ATmosphères EXplosives (ATEX) compliance. A key theme is the credibility and reliability of using Computational Fluid Dynamics (CFD) and other numerical methods in verification and approval processes.
Digital twins and advanced simulations offer great potential to improve understanding of process behavior and support documentation for regulatory approval. However, they also raise new questions: How do we ensure that a simulation is trustworthy? What are the best practices for model validation and uncertainty assessment? And how can digital verification be integrated into established quality systems and certification frameworks?
In this seminar, experts from industry and academia will present experiences, methods, and case studies on digital verification, validation of models, and the use of CFD for compliance documentation. Presentations will highlight how digital verification can reduce time-to-certification, enhance process safety, and improve decision-making.
Programme
| Time | Activity / Title | Description |
|---|---|---|
| 09:00 | Registration and Coffee | |
| 09:30 | Introduction and motivation Thomas Filholm, Aerotak |
|
| 09:45 | Pharmaceutical Qualification and Validation – Past, present and future Victor Bechmann, Pharmakon / Kuatro-group |
The talk starts with the Devonport accident and what it teaches about engineering responsibility. It then explains why qualification and validation are necessary, and outlines the structure and logic of the V-model. Next, it clarifies the stakeholder landscape — manufacturer, supplier, and authority — and their respective roles. The talk concludes with a forward-looking perspective on how CFD, design leveraging, and AI are changing qualification and validation practices. |
| 10:30 | Coffee break | |
| 11:00 | Validation of Cleanrooms Ulla Thomsen og Anne Rasmussen, Novo Nordisk |
A historical perspective of validation of cleanrooms |
| 11:30 | Virtual Temperature Mapping Joachim Toftegaard Hansen, Aerotak |
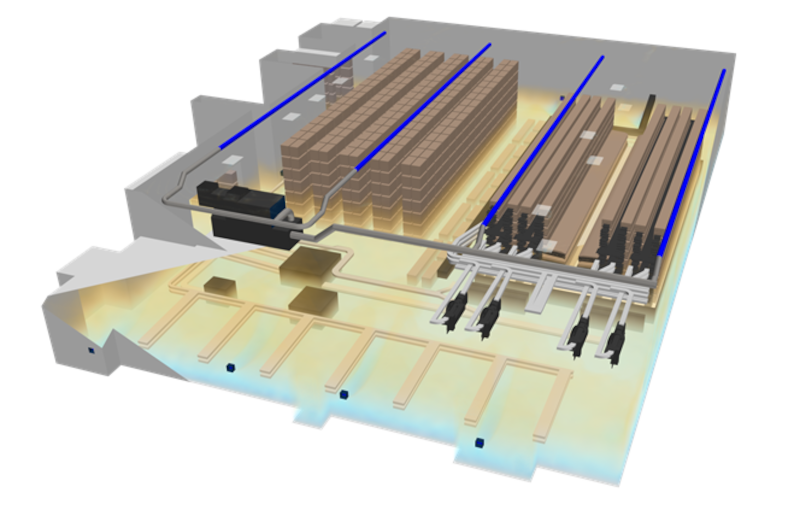
Temperature mapping is a critical part of qualifying temperature-sensitive spaces. In recent years, CFD has emerged as a powerful supplement to physical testing. This talk explores how virtual temperature mapping is used for qualification, highlighting the challenges, benefits, and potential pitfalls when simulations replace or reduce traditional test protocols. |
| 12:00 | Force Technology Rasul Niazmand Bilandi and Stephan Berger, Force Technology |
Marine Application |
| 13:00 | Gas Leak Hazards in Offshore and Hydrogen Systems Ingar Fossan, Chief Adviser, Safetec |
ATEX and Safety Considerations |
| 14:15 | Coffee and Cake Break | |
| 14:30 | Rambøll | |
| 15:00 | Front-loading V&V and regulatory compliance of aseptic process design using CFD Jenny K Lindblad and Ulf Lindblad, TetraPak |
|
| 15:30 | Open Slot | |
| 16:00 | Closing remarks Knud Erik Meyer, DANSIS chairman |
|
| 16:10 | General assembly |